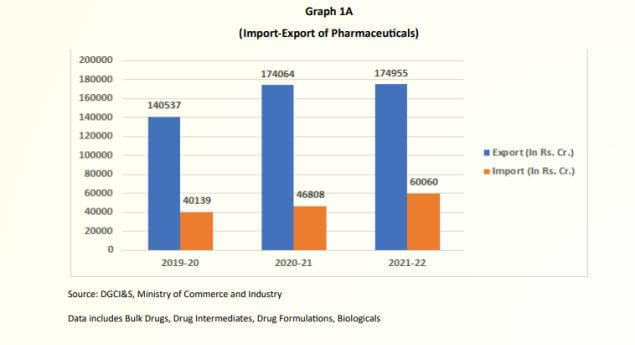
India’s pharmaceutical sector forms a major component of the country’s foreign trade and has been consistently making trade surplus as may be seen from the Graph 1A. During 2021-22, total exports value of pharmaceuticals products stood at Rs. 1,74,955 crore (USD 23.5 Bn) while value of imports were to the tune of Rs. 60,060 crore (USD 8.06 Bn) resulting in a surplus of trade value of Rs. 1,14,895 crore(USD 15.44 Bn).
Major challenge faced by this growing industry is regulatory compliance. Day in and day out, Pharma industry wakes up with a news that USFDA or a regulatory agency has issued import alert, warning letter, bunch of observations or findings. A snapshot at findings shows how serious is the concern.
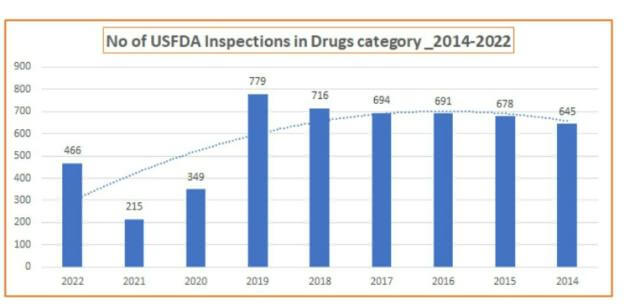
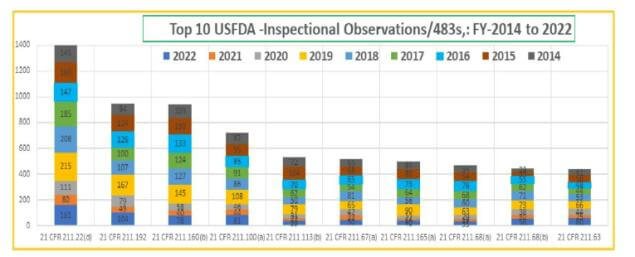
Recent past inspection data for last two years -2021 & 2022 shows an increase in the rate of observations, indicating 30-40% rise in the rate of observations per audit.
Overall review shows non-compliance in Quality system & Lab controls are the two areas being in focus in almost every regulatory inspection.
All sized pharma companies whether small, mid-sized or big are struggling with the issues with the regulatory agencies.
Risks Involved :
- Risk of Business Continuity
- Risk to Company’s Reputation
- Risk of ustainability in Pharma Industry
The above risks are threat to the business and a mitigation plan need to be in place.
KNORS suggests below risks mitigation plans:
- 24×7 readiness for the Regulatory Audits.
- Get your facility and systems assessed periodically by an independent and experienced consultant.
- Online review of investigations and CAPAs to be performed by the consultant.
- Train your team on critical thinking and technical writing.
- CAPA submitted to the Regulatory agency after the inspection decides the outcome of the audit.
Every industrialist tries to find a solution to this unavoidable happening. Question comes in mind is that even hiring best talent in the industry, why big organization face these challenges. Answer is visualizing the events/incidents as third eye to figure out the lacunas.
A knowledgeable and experienced consultant who has 360-degree view can identify the gaps and re-define the systems which will help organization to achieve long term goal.
KNORS Suggestions
- Get your facility and systems assessed by the experts who have experience of more than 500 audits.
- Assessment is by a tool which quantify the level of compliance; management can easily identify the weak areas and focus upon those.
- ON-line Review of the QMS data (Exceptions, Investigations and CAPAs); no fear before the regulatory inspection.
Contact Details:
Mail us at: drrajesh.kaushik@knorspharma.com
Call Us On: +91-9878880408



